Everywhere in North America, spring is in full bloom. Trees that had been barren for months sprout new leaves, old roots under the soil push up stems and grasses. Everything is suddenly green.
We take this incredible process for granted because it literally happens every year. But the way that plants feed themselves is miraculous and an advantage that the plant kingdom has always had over animals. Yes, we can move and stuff. But through the process of photosynthesis, plants can feed themselves. And they clean our air as they eat, too! Imagine if humans could do the same?
While we may not be able to feed ourselves this way anytime soon, we might be able to one day power our cities as plants do. Thanks to two chemists at the University of Illinois named Prashant Jain and Sung Yu, artificial photosynthesis power could be just around the corner!
Different than solar power
Green energy tech like solar and wind power work differently to this new idea. (Getty Embed)
In case this sounds like solar power, it's not. In solar power, light energy from the Sun is the thing that is turned into electricity. Instead, this new idea for artificial photosynthesis is modeled closely on how actual photosynthesis works in plants.
And how does that work again?
The perfect equation
The word 'photosynthesis' loosely means "using light to put together". (Getty Embed)
Photosynthesis begins with plants collecting water (H2O) and carbon dioxide from the air (CO2). On their own, these two things just kind of sit there. To get them to combine and change — synthesize — they need a catalyst, something to kickstart a reaction.
To do this, plants use a green pigment called chlorophyll to absorb light energy from the Sun. When exposed to this light energy, the water and carbon dioxide react and are turned into two things. The first of these — and the most important to the plant — are carbohydrates, aka sugars, aka food! The second thing? Oh, it's just some junk that the plant doesn't need. Oxygen!
This is why photosynthesis is one of the most important chemical reactions on Earth. It not only feeds plants efficiently (thus also providing food for countless animals), it also turns a toxic gas (CO2) into breathable air.
Turning CO2 to fuel
Both chlorophyll and the gold nanoparticles in this experiment collect green light from the light spectrum. (Getty Embed)
Now imagine a big power station that did the same thing.
That's basically what the experiments that Jain and Yu have conducted are all about. Instead of chlorophyll, they use gold nanoparticles as their catalyst. It also absorbs the same kind of light energy, then uses it to get a reaction between water and CO2. Their result though is not sugars — it is a liquefiable hydrocarbon fuel. And again, oxygen is a by-product. Cool!
Doesn't have the green light (yet)
To become the future of energy, this new fuel needs to be used differently than this. (Getty Embed)
As exciting as this is, there are a couple of reasons why it's not getting the (haha) green light right away.
First is scale, or size. Right now, this is just an experiment in a lab. Turning it into a device that is large and efficient enough to power cities? That's going to take some time.
Second? This hydrocarbon fuel is still a fuel. (It is essentially propane, a fuel used in barbecues and some vehicles.) The easiest way to use it, is to burn it... a chemical reaction that creates more CO2. And that kind of defeats the purpose of using artificial photosynthesis to clean the air as we make energy!
The good news? There are labs around the world that are right now figuring out greener ways to convert this sort of fuel directly into electricity. If scientists can figure these problems out, we could one day have cities that breath and "eat" as a large forest does.
As a green energy future, that's mind-boggling!
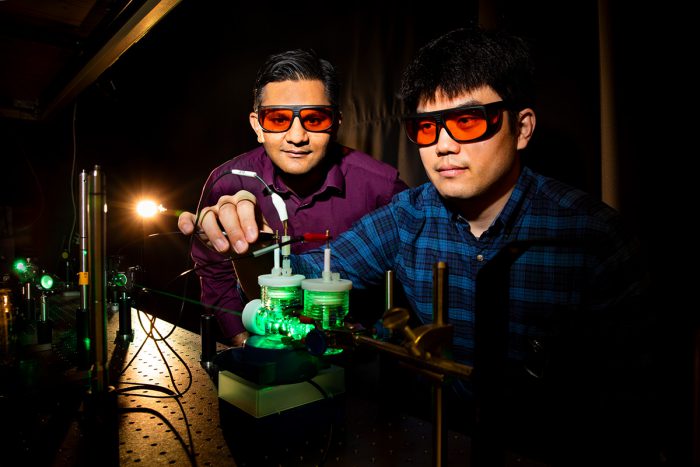 Professor Prashant Jain, left, and research associate Sungju Yu have made a breakthrough that could change green energy forever. (University of Illinois/Fred Zwicky)
Professor Prashant Jain, left, and research associate Sungju Yu have made a breakthrough that could change green energy forever. (University of Illinois/Fred Zwicky)










I’m learning about photocinthisis I school right now!
?????☘?????⚘??