Chemists are wizards.
Most don't have long beards, high-peaked hats, or flowing robes. But whatever magic is in the real world, chemistry is as close to it as we can get to it. Chemists use their deep understanding of the molecules and atoms that make up our universe to understand what will bind, what will repel, what will melt, what will freeze. And yes, what will explode!
They also understand how to synthesize—or create—brand new materials. One of the latest of these is borophene (say BOR-oh-feen), and it could start appearing in a bunch of things that we use everyday like batteries and sensors.
Sorry, are we boron you?
Say hi to boron. (© Blueringmedia - Dreamstime.com)
Let's start with the element that borophene is made from. Boron.
You can find boron next to some pretty common and essential elements on the periodic table, such as carbon, nitrogen, and oxygen. It is known as a metalloid—this means that it is somewhere between metals like iron and tin and non-metals like oxygen or sulphur.
So why don't we hear about it that much? For one, it is pretty rare. And so far, it hardly has a starring role in our everyday lives. Sure, you can find boron as an additive in things like insulation and high-strength glass, but you kind of need to know where to look for it.
The invention of borophene, however, could put boron into the spotlight.
What a boro-find!
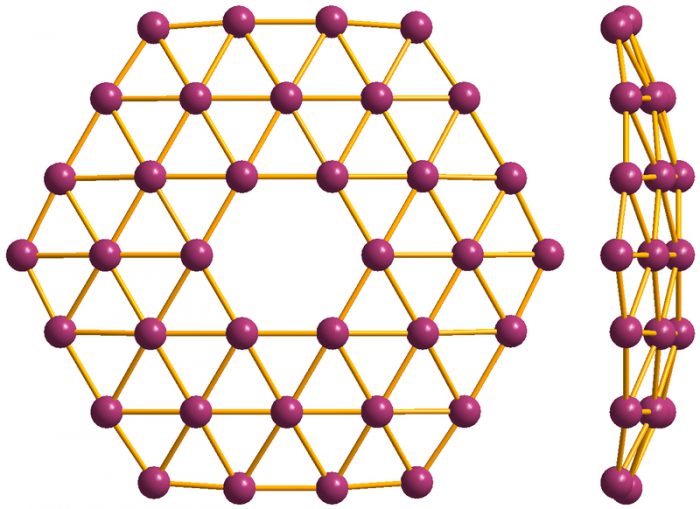
This image shows how the atoms in a sheet of borophene are laid out. (Materialscientist/Wikimedia Commons)
Borophene is a very thin layer of boron atoms that is made to form crystalline structures. It was only first created by chemists in 2015. They made it by forcing hot boron gas to condense on a layer of chilled pure silver. Why pure silver? Turns out that as it cools on the surface, the boron atoms imitate the regular arrangement of the silver atoms—almost like pouring hot metal into a mold and letting it cool into a shape. Only on a microscopic, atomic level!
Basically, you get a thin sheet of boron that is lightweight, mega-strong, ultra-flexible, and an excellent conductor of electricity and heat. (A conductor allows energy to travel through it.) In a nutshell, chemists dream of creating these sorts of materials because they can have many uses.
Borophene could improve the performance of lithium-ion batteries. It could be used to improve sensors, too. And it also has a bunch of potential applications in water-based energy.
It is also very expensive to make, so a borophene future isn't exactly around the corner. But this news is a reminder of how, well, magical the field of chemistry can be. If you want to be a real life Harry or Hermione, the school science lab might be the best place to start!
 Borophene is made from this element, boron. (James L. Marshall/Wikimedia Commons)
Borophene is made from this element, boron. (James L. Marshall/Wikimedia Commons)










boring worst article ever