Gold. Most people think of it as a sign of wealth. Power. Prestige.
But to a chemist, gold is another thing entirely. It is a catalyst. What's that?
A catalyst is something that helps to start chemical reactions, like lighting a wick on a firecracker. Except, not all catalysts lead to explosions. They lead to change.
When gold nanoparticles (microscopic pieces) are added to certain elements, amazing things can happen. For example, gold can turn carbon monoxide (CO) into carbon dioxide (CO2). Back in May, we wrote about a photosynthesis process that turns CO2 into fuel ... and gold is the catalyst there, too!
And then there's this unreal experiment where a liquid filled with gold nanoparticles goes from being a window to a mirror whenever an electrical current is applied! (Just wait for the reveal at the end ... amazing!)
So bottom line: gold is scientific, well, gold! And now, we'd like to tell you about 'nanoseaweed'.
Wait, what?
Gold leaf
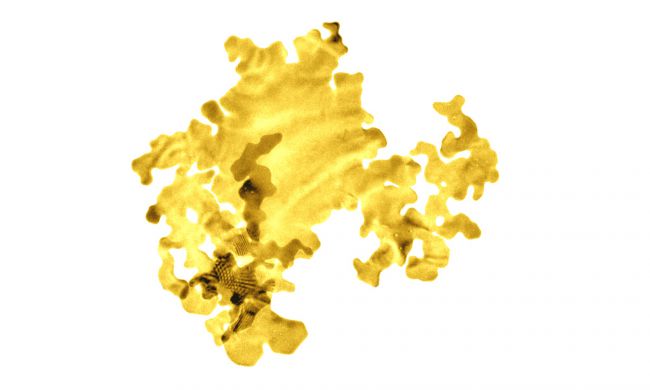
A piece of nanoseaweed. (University of Leeds)
Don't worry, we're still talking about gold and chemistry here. Nanoseaweed is not actually algae, though it looks a lot like a leaf. It's a name given to a new experimental from of gold 'grown' by a group of scientists at the University of Leeds, England.
It is the thinnest gold in the world—just 2 atoms thick. That's so thin, it makes the thinnest razor seem as thick as a concrete wall.
Why is this important? Does it relate to gold being a catalyst at all?
Ah ha! Now you're talking!
On the surface
Any chemical reaction is about stuff happening at an atomic level—atoms and molecules coming together. The hottest thing in our solar system (spoiler alert: it's the Sun!) is powered by trillions of atomic reactions every second. And the more molecules that are involved, the more powerful and efficient the reaction becomes.
This fact is what makes nanoseaweed so exciting. By stretching the gold so thin, it means that nearly every single particle of gold is exposed to the surface of the leaf. And this means that basically all of the gold participates in a catalytic reaction.
In experiments, nanoseaweed is able to produce efficient, speedy chemical reactions, meaning it could have a big future wherever gold is used today in technology and medicine.
So what have we learned? In society, the thicker the gold bar, the better. But in science? A little nanoseaweed goes a loooooong way!
 Scientists think that these exceptionally thin pieces of gold could be very useful. (University of Leeds)
Scientists think that these exceptionally thin pieces of gold could be very useful. (University of Leeds)










Wow